pH Specification
In WAVE, pH is defined as –log10 of the H+ concentration (in mol/L). For a solution of fixed composition, the H+ concentration and thus the pH will be a function of the temperature due to the temperature dependence of equilibrium constants. As an example, the ionization constant of water is a function of temperature, with pK values of 14.94, 14.00, and 13.26 at 0, 25, and 50 °C, respectively. This implies that the pH of pure water is 7.47, 7.00, and 6.63 at 0, 25, and 50 °C, respectively. The equilibrium constants for carbonate alkalinity reactions
and ammonia reactions
are also functions of temperature. Thus the concentrations of all of these species will be a function of pH and temperature.
In WAVE, when pH is given as an input, it is assumed that this corresponds to the H+ concentration at the design temperature. If the solution is charge balanced by adding solutes or adjusting solutes, the H+ concentration at the design temperature is fixed. Only if the solution is charged balanced by adjusting pH will the H+ concentration at the design temperature vary. After a solution is charge balanced, WAVE displays on the feed water screen the pH at both the design temperature and at a standard temperature of 25 °C.
Once you have a charge balanced solution at the design temperature, if a computation is run at a different temperature then WAVE will automatically determine the correct pH for that temperature and adjust the water chemistry appropriately. This occurs when the user selects a computation temperature on the RO screen or the summary screen other than the design temperature (for example, minimum or maximum).
In some cases the pH is known at one temperature, but the design is to be run at a substantially different temperature. This commonly occurs in condensate polishing, where the pH at 25 °C is known but the system design temperature is substantially higher. When this situation occurs, the following protocol is recommended to get the most accurate results.
- Initially determine the water composition at the temperature where the pH is known.
- Enter the known pH.
- Enter the known pH temperature (25 °C) as the design temperature.
- Enter the remaining constituents of the feed water.
- Charge balance the solution using one of the Add Solutes or Adjust Solutes options.
- Determine the water composition at the design temperature
- Enter the actual design temperature
- Charge balance the solution using the Adjust pH option. The total moles of related species will be held constant, but all of the equilibrium constants will be recomputed for the new temperature and then the H+ concentration and thus the pH will be adjusted to charge balance the solution.
As an example, assume that the pH of an ammonia solution is known to be 9.50 at 25 °C. Inputting those values and using the Add Ammonia option gives a total NH3/NH4+ concentration of 1.615 mg/L as NH4+. If a design temperature of 55 °C is entered, using the Adjust pH option will give a pH at that temperature of 8.65. Note that the pH at 25 °C is correctly displayed as 9.50.
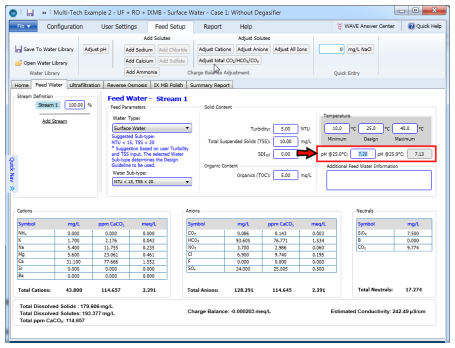
Figure 1: pH entry