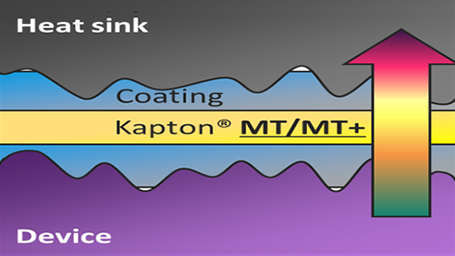
Extreme Versatility and Thermal Performance Provides Unlimited Potential
From the circuits in the cameras on space missions to the next generation of photovoltaic cells, DuPont™ Kapton® polyimide films are helping make extraordinary new design possibilities actually happen.
For applications where extremes of heat and vibration are the norm, designers rely on Kapton® because of its ability to maintain its unique combination of mechanical properties under the harshest of conditions.
DuPont™ Kapton® polyimide films have set the industry standard for over 45 years in high performance, reliability and durability, with a unique combination of electrical, thermal, chemical and mechanical properties that withstand extreme temperature, vibration and other demanding environments.
Polyimide Film Products
-
Kapton® Polyimide films
Kapton® Polyimide films
Maintains its unique combination of mechanical properties under the harshest of conditions.
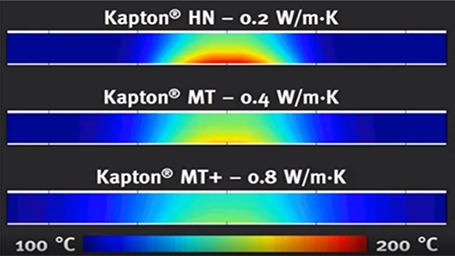
This matte black polyimide film offers exceptional physical, chemical, and electrical properties in a broad temperature range, as well as excellent dimensional stability at high temperatures.This high-quality polyimide film is engineered to withstand the damaging effects of coronal partial discharge associated with high voltage AC electrical designs and equipment. Its durability and resistance make it an ideal choice for optimal protection.This premium polyimide film is ideal for use as a dielectric substrate in flexible printed circuits and high-density interconnects. It offers superior stability, a high modulus, and a matching coefficient of thermal expansion to copper, making it the preferred choice for very fine pitch circuitry.Superior quality polyimide films for use as a dielectric substrate. A perfect choice for chip on film, flexible printed circuits, high-density interconnects, and next-generation IC packaging applications. These films offer excellent CTE control, high modulus, and surface quality, making them preferred dielectric films.This innovative polyimide film is designed for high-reliability applications that require resistance to corona partial discharge in fast-switching AC electrical designs. With FEP fluoropolymer applied, Kapton® FCRC enables bonding in spiral-wrapped conductor insulation designs for motor and magnet wire, making it an ideal choice for various applications.Designed with FEP fluoropolymer on one or both sides of Kapton® HN, this polyimide film is durable, versatile and offers added chemical resistance. Its heat bonding and sealing capabilities make it ideal for a range of industrial applications.This polyimide film is treated on both sides and features exceptional physical, chemical, and electrical properties over a wide temperature range, similar to our general-purpose Kapton® film. Additionally, it provides superior dimensional stability and adhesion specifically designed for flex circuit manufacturers who require high-quality adhesion and low shrinkage properties.This polyimide film includes a white pigmented FEP fluoropolymer applied on one or both sides of Kapton® HN, providing added chemical resistance and heat bonding or sealing capabilities for a diverse range of industrial applications.For enhanced hydrolytic stability and superior heat bonding/sealing abilities for industrial use, choose Kapton® FWR polyimide film. This material offers a more reliable and long-lasting alternative to traditional polyimide fluoropolymer composite films.For applications requiring a high-performance all-polyimide film, Kapton® HN is the recommended choice due to its exceptional balance of properties over a wide temperature range. It has proven effective in extreme temperatures, ranging from -269°C to 400°C. This versatile film can be laminated, metallized, punched, formed, or coated with adhesives.This high-performance polyimide film is treated on both sides and features similar physical, chemical, and electrical properties as general-purpose Kapton® HN across a broad temperature range. It offers superior dimensional stability and adhesion, making it an excellent choice when low shrinkage and exceptional adhesion are critical factors.The Kapton® MT polyimide film family offers an enhanced thermal conductivity of 0.45W/mK compared to traditional polyimide films. It combines the electrical properties, thermal conductivity and mechanical toughness to control and manage heat in electronic assemblies.Kapton® MT+ polyimide film family offers over 4x improved thermal conductivity with 0.8W/mK compared to traditional Kapton® films while maintaining superior mechanical, electrical, and thermal properties. Kapton® MT+ sets the industry standard for thermal conductivity performance in the polyimide category.This polyimide film composite with fluoropolymer is intended for severe-duty magnet wire and motor applications and provides improved resistance against scrape abrasion, as well as higher operating temperatures.This tough polyimide film offers an excellent balance of physical, chemical, and electrical properties at high temperatures. It is specifically engineered for use in the pressure-sensitive tape industry, providing improved characteristics for PST coaters, including better film attributes and crystal structure.For heating applications requiring lightweight and uniform heating, Kapton® RS electrically conductive polyimide film offers tightly controlled 100 ohms/sq surface resistivity. The resistive property is an inherent feature of the film, providing long-lasting durability and resistance to damage from cracking or rubbing.This polyimide film features a proprietary fluorocarbon resin coating on one or both sides of Kapton® HN polyimide film. With excellent adhesive properties and mechanical strength at high temperatures, it outperforms Kapton® FN polyimide films. -
Important Information
Important Information
This list of literature below is not specific to one product grade
We’re here to help.
We love to talk about how our electronics solutions can build business, commercialize products,
and solve the challenges of our time.