Uncovering advantages with digital printing on healthcare packaging
While helping to meet UDI requirements, digital printing has many benefits such as improving production efficiencies, increasing flexibility, reducing waste and improving patient outcomes
With the full adoption of UDI requirements in the US FDA and the EU MDR, traceability of medical devices will be significantly enhanced. Better traceability leads to improved incident reporting, targeted field safety corrective actions and better monitoring by components authorities, as described in the regulation. Ultimately, increased transparency and access to information will protect public health and enable informed decisions to be made by the healthcare professionals to the benefit of patients.

Enabling better barcodes
Barcodes provide a practical way to store all of the required data into a small space and enable quick access to regulatory databases for reliable identification and traceability. To help support patient safety, it is crucial that the printed barcodes work well within their intended use environment, consisting of manufacturing, possible sterilization, distribution and storage until the point where the product is used. Please see also the white paper “Printing variable data on DuPont™ Tyvek® for healthcare packaging” in the online DuPont Resource Center.

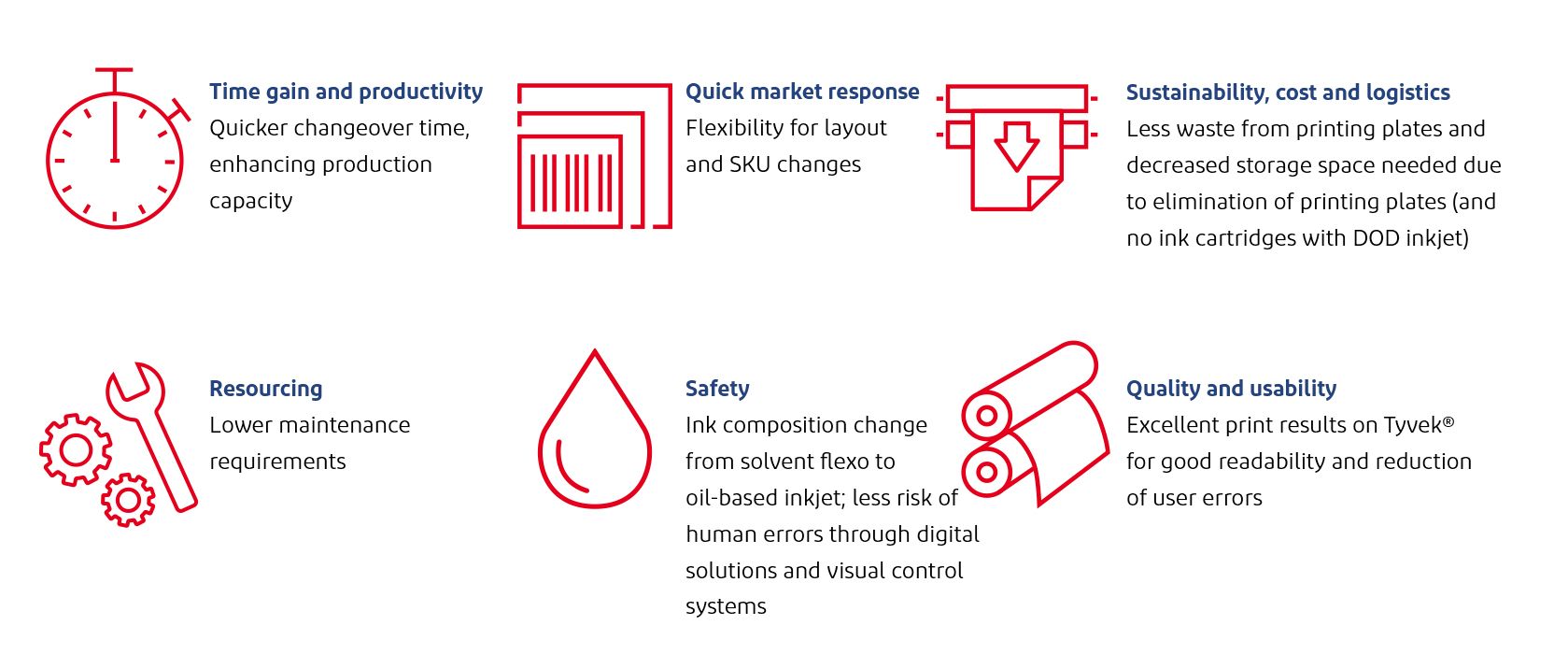
Vygon evaluates printing options
Procedure packs, or medical device kits, contain a combination of products packaged together for a specific medical purpose. Kits can contain several medical devices or components nested in a package such as a blister tray for easy and safe access during a medical treatment. Vygon designs, manufactures and markets high-tech single-use medical devices for healthcare professionals. This article focuses on peripherally inserted central catheters from Vygon that are supplied in a kit tray consisting of four or more components: catheter, sheath, needle and guidewire. The PETG tray is thermoformed on a form-fill-seal line followed by the manual placement of devices into the formed tray and the sealing with a lid made of coated Tyvek® 2FS™. For components contained inside the hard blister tray, Tyvek® 2FS™ provides trusted performance against punctures and other threats that could compromise sterile integrity.
Vygon switches to digital printing
“Due to its specialty products, Vygon has a very wide range of different sets which require a different print image; however, the lot sizes are comparatively small,” Volker Ganser, Technical Manager at Vygon, explained. “It is therefore important to reduce setup times and avoid downtime and rework. With the switch to digital printing, manual printing plate changes by employees are replaced by an automated digital process acting when there is an order change on the packaging machine.”