Superior Microbial Barrier protects your device’s sterility
DuPont™ Tyvek® Healthcare Packaging
Access the 2023 Medical Packaging Conference virtual event replays and learn more about Designing for recycling and sustainability in healthcare packaging.
Medical Packaging Conference 2025 - Register Today

Registration is now open for the 2025 Medical Packaging Conference, taking place 6-8 October in Frankfurt, Germany.
Join healthcare and healthcare packaging industry leaders for an in-person look at what’s shaping the future of healthcare packaging, from regulatory changes and sustainability advancements to transportation, recycling and clinical challenges. The 2025 Tyvek® Sustainable Healthcare Packaging Award winners will also be announced.
Don't miss out on the early bird rate! Please explore the agenda and register to attend.
No Compromise. Because life matters.
DuPont™ Tyvek® Healthcare Packaging provides the tear resistance, durability, breathability, clean peel and superior microbial barrier to keep medical equipment and pharmaceuticals sterile throughout their lifecycle—protecting the health and well-being of millions without compromise.
Unlike medical-grade papers and films, Tyvek® is compatible with all of the most commonly used sterilization methods, including:
- Ethylene Oxide (EO)
- Radiation (Gamma and electron beam)
- Steam under controlled conditions
- Low-temperature oxidative
Tyvek® has been a trusted choice for medical device and pharmaceutical manufacturers as well as specialized sterile packaging suppliers worldwide for decades. As an important extension of that role, we are actively working to advance sustainability across the healthcare packaging value chain.
Tyvek®— Advancing sustainability in healthcare packaging
Making healthcare packaging sustainable is a challenging journey that requires sustainable innovation, continuous improvement and proactive collaboration. We are taking actions to meaningfully advance sustainability across the healthcare packaging value chain.
Products for Healthcare Packaging
Tyvek® 1073B
Tyvek® 1073B provides the highest level of protection, making it the ideal choice for medical device and pharmaceutical packaging that requires high strength and superior microbial barrier.
Tyvek® 1073B
Explore
Tyvek® 1059B
Tyvek® 1059B provides robust protection for medium-risk, sterile medical packaging applications. Lighter weight than Tyvek® 1073B, this proven performer is ideal for smaller devices and those with rounded edges.
Tyvek® 1059B
Explore
Tyvek® 2FS™
Tyvek® 2FS™, a high puncture-resistant substrate, outperforms paper in terms of toughness and microbial barrier. A light-weight form of Tyvek®, it is well suited for form-fill-seal, as well as cost-sensitive applications requiring strength.
Tyvek® 2FS™
Explore
Products for Pharmaceutical In-Process Applications
Tyvek® 1073B
Tyvek® 1073B provides the highest level of protection, making it the ideal choice for medical device and pharmaceutical packaging that requires high strength and superior microbial barrier.
Tyvek® 1073B
Explore
Tyvek® 1421B
Tyvek® 1421B is specifically developed for in-process pharmaceutical applications to protect surfaces of cleanroom equipment, components and accessories from particle and microbial contamination.
Tyvek® 1421B
Explore
Tyvek® with Renewable Attribution
Tyvek® with Renewable Attribution is an extension of our existing Tyvek® Healthcare Packaging portfolio that offers a significantly reduced carbon footprint.
Tyvek® Sustainable Healthcare Packaging Awards
We’re proud to announce a new award program that recognizes and encourages advancing sustainable innovation throughout the entire healthcare packaging value chain.
Forward Together™
By asking, listening, and collaborating with our customers every step of the way, the DuPont Tyvek® team is able to create trusted, sustainable healthcare packaging that benefits our customers, patients, and all of us. Now and into the future, let’s move Forward Together™.
Featured Information
Where to Buy
See our list of Authorized Converters by region
Where to Buy
Explore
Resource Center
Explore Tyvek® through various technical and regulatory documentation
Resource Center
Explore
Film finder tool
Film finder tool
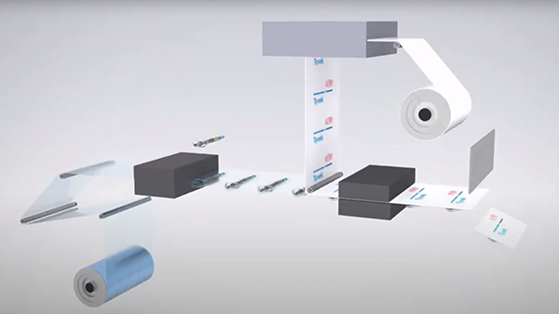
Uncovering advantages with digital printing on healthcare packaging
While helping to meet UDI requirements, digital printing has many benefits such as improving production efficiencies, increasing flexibility, reducing waste and improving patient outcomes