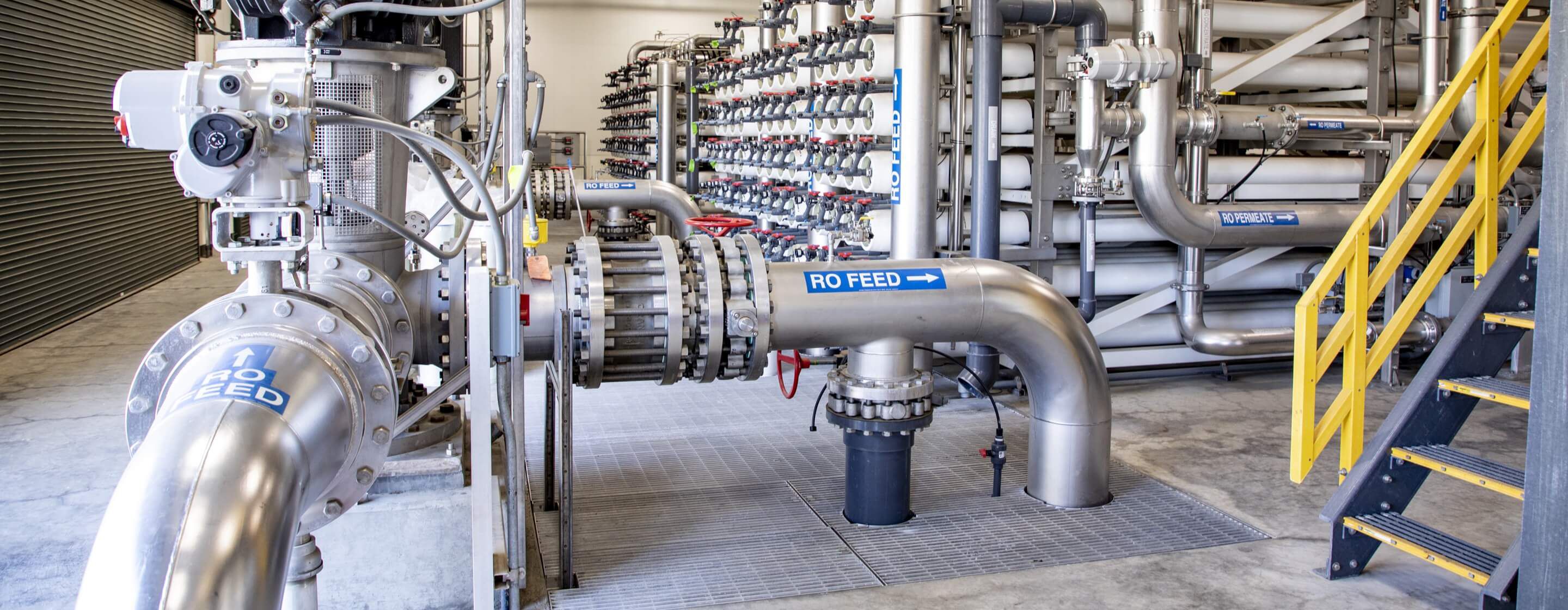
Proven, reliable, high-performance water purification
Compared to traditional filtration technologies that rely on a screen or filter to remove particles, reverse osmosis (RO) is a pressure-driven separation process that employs a semipermeable membrane and the principles of crossflow filtration.
Reverse osmosis water treatment provides the finest level of filtration. The RO membrane acts as a barrier to all salts and inorganic molecules, as well as organic molecules with a molecular weight greater than approximately 100. It is therefore a highly effective process for removing contaminants such as:
- Endotoxins/pyrogens.
- Insecticides/pesticides.
- Herbicides.
- Antibiotics.
- Nitrates.
- Sugars.
- Soluble salts.
- Metal ions.
We provide the most widely used RO technology in the world — trusted globally by municipalities , industries , manufacturers, commercial markets, and families wanting clean, healthy water at home.
A closer look
Overcoming osmotic pressure
In ordinary osmosis, when a semipermeable membrane separates solutions of differing solute concentrations, the lower-concentration solution flows into the higher-concentration solution in attempt to reach equilibrium: an equal degree of solute concentration on both sides of the membrane. As the amount of solution on the higher-concentration side increases, pressure on that water column rises until it is high enough to hinder the flow of the lower-concentration solution across the membrane. This is the action of osmotic pressure.
In reverse osmosis (RO), pressure that exceeds a system’s osmotic pressure is applied to that system. The pressure forces the higher-concentration solution back across the semipermeable membrane, leaving solutes that are blocked by the semipermeable membrane behind.
Typically, reverse osmosis water treatment results in a rejection of dissolved salts that is 95 – 99 percent or greater, depending on membrane type, feed composition, temperature, and system design.
Reverse osmosis water treatment can provide finer filtration than either nanofiltration or ultrafiltration. Using RO as a pretreatment process for ion exchange (IX) can substantially reduce the operating costs and regeneration frequency of the IX system.
Typical applications include:
- Purification of home drinking water.
- Desalination of seawater or brackish water to produce drinking water.
- Wastewater recovery.
- Food and beverage processing.*
- Biomedical separation.
- Industrial process water treatment.
Reverse osmosis water treatment is also often used to produce ultrapure water for the semiconductor industry , in boiler water treatment for the power industry, and for applications in the health care and bioprocessing industry.
Maintaining flow and desired results
Precipitate salts and other impurities that a pressurized flow of feedwater forces against a semipermeable RO membrane can clog or “foul” that membrane. This, in turn, can decrease the performance of a reverse osmosis water treatment system overall.
To significantly reduce the rate of membrane fouling, RO elements use crossflow filtration. This process forces lower-concentration water through the RO membrane, while the separated flow of higher-concentration water moves across the surface of the membrane, carrying away the rejected salts and impurities.
In essence, crossflow filtration happens like this: A high-pressure pump continuously pumps feedwater into the element of the reverse osmosis water treatment system. The pressure forces some water to cross the semipermeable RO membrane, resulting in a low-saline or purified product called permeate on one side, and a high-saline or concentrated brine, called concentrate or reject, on the other. A concentrate valve controls the percentage of feedwater that goes to the concentrate stream and the permeate. In the system, the low-saline or purified permeate — the feedwater that has passed through the membrane — remains isolated from the concentrate flow. The concentrate stream removes the concentrate that cannot permeate the membrane and sweeps them out of the system.
Reverse osmosis systems can thus:
- Produce purified water (or permeate) from a feed stream or brine.
- Remove a concentrate (or concentrated brine or reject) from a feed stream.
Depending on the need and application, either the permeate or the concentrate may be the desired product.
Reverse osmosis water treatment can also be used to selectively separate certain ions and molecules, although to a lesser degree than can ion exchange systems.
Influencing factors
RO membrane performance (improvement or degradation) is affected by a number of different factors, including aspects of the feedwater, such as:
- Temperature.
- pH balance.
- Salt concentration.
Normalization calculation tools can help distinguish between the normal, predictable performance changes caused by factors such as those listed above, and deteriorating performance caused by membrane fouling or similar issues.
Other factors influencing RO membrane performance include:
- Operations parameters such as system recovery.
- Concentration polarization.
Find more details in our tech fact sheet: FILMTEC™ Membranes: Factors Affecting RO Membrane Performance
-
What Is RO?
Overcoming osmotic pressure
In ordinary osmosis, when a semipermeable membrane separates solutions of differing solute concentrations, the lower-concentration solution flows into the higher-concentration solution in attempt to reach equilibrium: an equal degree of solute concentration on both sides of the membrane. As the amount of solution on the higher-concentration side increases, pressure on that water column rises until it is high enough to hinder the flow of the lower-concentration solution across the membrane. This is the action of osmotic pressure.
In reverse osmosis (RO), pressure that exceeds a system’s osmotic pressure is applied to that system. The pressure forces the higher-concentration solution back across the semipermeable membrane, leaving solutes that are blocked by the semipermeable membrane behind.
Typically, reverse osmosis water treatment results in a rejection of dissolved salts that is 95 – 99 percent or greater, depending on membrane type, feed composition, temperature, and system design.
Reverse osmosis water treatment can provide finer filtration than either nanofiltration or ultrafiltration. Using RO as a pretreatment process for ion exchange (IX) can substantially reduce the operating costs and regeneration frequency of the IX system.
Typical applications include:
- Purification of home drinking water.
- Desalination of seawater or brackish water to produce drinking water.
- Wastewater recovery.
- Food and beverage processing.*
- Biomedical separation.
- Industrial process water treatment.
Reverse osmosis water treatment is also often used to produce ultrapure water for the semiconductor industry , in boiler water treatment for the power industry, and for applications in the health care and bioprocessing industry.
-
Crossflow Filtration
Maintaining flow and desired results
Precipitate salts and other impurities that a pressurized flow of feedwater forces against a semipermeable RO membrane can clog or “foul” that membrane. This, in turn, can decrease the performance of a reverse osmosis water treatment system overall.
To significantly reduce the rate of membrane fouling, RO elements use crossflow filtration. This process forces lower-concentration water through the RO membrane, while the separated flow of higher-concentration water moves across the surface of the membrane, carrying away the rejected salts and impurities.
In essence, crossflow filtration happens like this: A high-pressure pump continuously pumps feedwater into the element of the reverse osmosis water treatment system. The pressure forces some water to cross the semipermeable RO membrane, resulting in a low-saline or purified product called permeate on one side, and a high-saline or concentrated brine, called concentrate or reject, on the other. A concentrate valve controls the percentage of feedwater that goes to the concentrate stream and the permeate. In the system, the low-saline or purified permeate — the feedwater that has passed through the membrane — remains isolated from the concentrate flow. The concentrate stream removes the concentrate that cannot permeate the membrane and sweeps them out of the system.
Reverse osmosis systems can thus:
- Produce purified water (or permeate) from a feed stream or brine.
- Remove a concentrate (or concentrated brine or reject) from a feed stream.
Depending on the need and application, either the permeate or the concentrate may be the desired product.Reverse osmosis water treatment can also be used to selectively separate certain ions and molecules, although to a lesser degree than can ion exchange systems.
-
RO Membrane Performance
Influencing factors
RO membrane performance (improvement or degradation) is affected by a number of different factors, including aspects of the feedwater, such as:
- Temperature.
- pH balance.
- Salt concentration.
Normalization calculation tools can help distinguish between the normal, predictable performance changes caused by factors such as those listed above, and deteriorating performance caused by membrane fouling or similar issues.Other factors influencing RO membrane performance include:
- Operations parameters such as system recovery.
- Concentration polarization.
Find more details in our tech fact sheet: FILMTEC™ Membranes: Factors Affecting RO Membrane Performance
Our approach to reverse osmosis water treatment
A critical component of the FilmTec™ family of products, DuPont Water Solutions reverse osmosis water treatment technology supports high-quality, top-performing water purification in consumer, commercial, municipal, and industrial water treatment systems across the globe.
Our FilmTec™ RO elements house spiral-wound, thin-film composite polyamide membranes. Typically, a spiral-wound configuration means lower replacement costs, simpler plumbing systems, easier maintenance, and greater design freedom than other configurations, such as tubular, plate-and-frame, and hollow-fiber module designs. Our RO elements are manufactured using the most highly advanced, automated manufacturing technology in the industry, which results in maximum uniformity and minimized element-to-element differences. Our rigorous, extensive quality testing ensures that FilmTec™ elements meet our high fabrication standard.
Compared to cellulose membranes, our FilmTec™ elements for reverse osmosis water treatment can:
- Produce two to three times more high-quality water.
- Screen out a higher percentage of dissolved solids.
- Reject a higher percentage of undesirable dissolved solids, including chloride, lead, and nitrates.
Our high-performance FilmTec™ RO elements for commercial markets — such as for car washes, hotels and resorts, and hospitals and other institutions — as well as municipal and industrial markets, can:
- Remove up to 99 percent of impurities from water.
- Reduce operating costs.
- Lower energy usage.
- Provide longer life due to antifouling and high cleanability.
Find reverse osmosis products
View a list of our industry-leading reverse osmosis products.
Related industries
Our technologies provide premium solutions for a broad range of industries. Learn more about the industries that depend on reverse osmosis technology.

We help offices, schools, hospitals, hotels, and universities enhance their facilities with our water-treatment solutions.

We help everyone’s favorite foods and beverages taste better with advanced separations of sugars, dairy streams, and other nutritional ingredients.

Our technologies and solutions are designed to help you overcome water challenges to produce your desired quantity and quality of industrial utility water.

We develop best-in-class technologies, accompanied by an advanced product portfolio of solutions, to address your crucial wastewater challenges.
We enable the production of some of today’s most popular technologies by facilitating ultrapure water and effective water reuse.

We help ensure a steady flow of clean, safe drinking water into local communities with our water-treatment solutions

We help energy companies improve operational efficiency with specialized water treatment and wastewater reuse.

We enable the smooth and safe operation of power plants through innovative water-treatment and wastewater reuse.

We help improve the safety and quality of drinking water in homes with exceptional water treatment
Related resources
Notes
*Reverse osmosis membranes have received FDA clearance for use in processing liquid foods and in purifying water for food applications. This clearance is published in the Code of Federal Regulations under Title 21, Section 177.2550, Reverse Osmosis Membranes. Our FT30 reverse osmosis membrane complies with this regulation.
See what’s possible
Ask us how reverse osmosis can help you achieve high-quality, top-performing water purification.