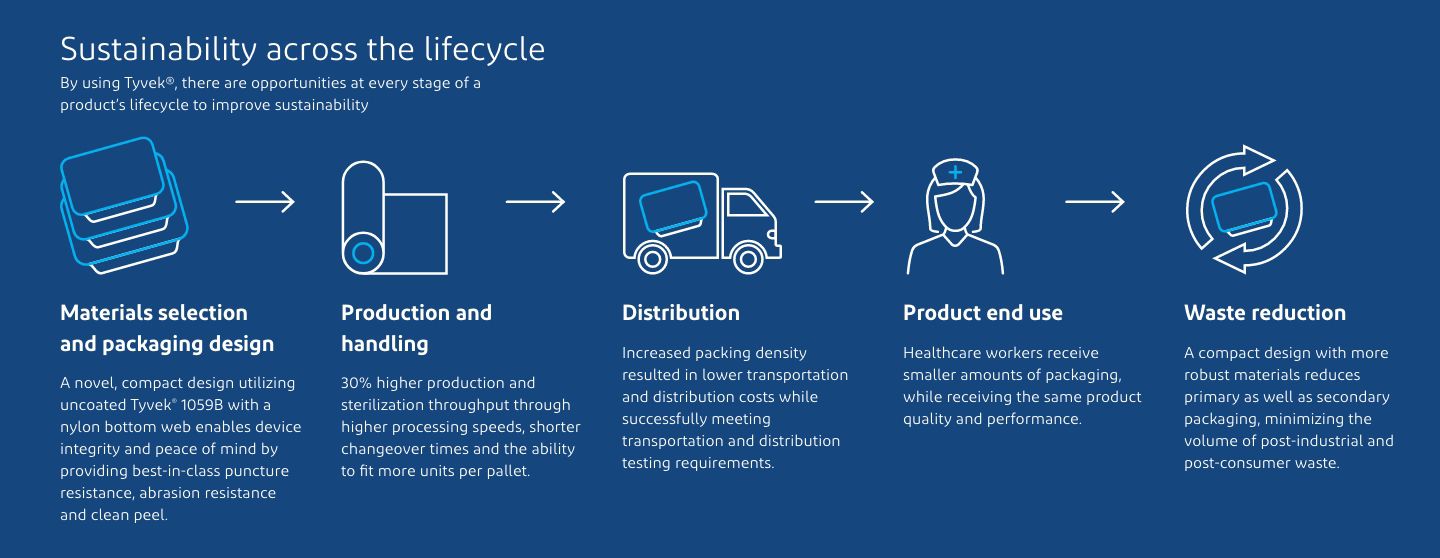
Enabling sustainable packaging design
A collaboration between B. Braun and the Tyvek® team
In collaboration with B. Braun Medical Inc. (B. Braun), a leader in infusion therapy and pain management, DuPont is proud to contribute to the development of packaging that enables responsible consumption and production practices (UN SDG #12). Aided by the use of DuPont™ Tyvek® in their medical device packaging, this case study provides an example of how B. Braun has driven exceptional operational efficiency and sustainability gains across the lifecycle of their products.
Guided by B. Braun’s core philosophy of Sharing Expertise® and a long-standing focus on sustainability, B. Braun exemplifies environmental responsibility in virtually all facets of product development, manufacturing and delivery. “We look for every opportunity to reduce our environmental footprint, and packaging is one of the areas where we can have a lasting impact across the supply chain,” said Damian Rodriguez, Director of Package Labeling and Product Identification for B. Braun. Similarly, DuPont is committed to meaningfully advancing the UN SDGs of responsible consumption and production and good health and well-being through sustainable innovation, continuous improvement and partnerships to enable a circular economy with solutions like Tyvek®.
Through this project, B. Braun and the Tyvek® team strived to leverage the importance of the role played by packaging designs and materials selection in sustainability, and the opportunities this presents in improving operational efficiency and the environmental footprint of sterile medical device production.
Damian Rodriguez, Director of Package Labeling and Product Identification, B. Braun
The challenge
B. Braun’s IV sets are used for the delivery of different infusion therapies, including nutritional, pain management, renal anesthesia and fluid replenishment in general. These products are paired with other B. Braun devices, such as IV tubing, pumps and bags. Specifically, B. Braun aimed to optimize the design and packaging of their infusion sets to minimize waste and reduce the environmental footprint. The infusion sets presented unique challenges for the packaging design team – for instance, the low modulus, low mass IV tubing which is soft and light, did not present friction or puncture risks to the package, but needed to be coiled to an efficient size and in a manner that did not kink the tubing, a critical requirement for the device. In addition to the tubing, the devices contain rigid components - needle-free ports, connectors, clamps and other hard plastic components, presenting abrasion and puncture challenges. This assembly of soft, long, alternatively rigid and potentially sharp materials all need to be encapsulated in a flexible package. Achieving this balance necessitated a design and material selection process that enabled efficient package sizing, while not compromising patient safety and sterile integrity. Furthermore, the package design needed to be adaptable to the entirety of B. Braun’s infusion set portfolio, comprising hundreds of different SKUs, differentiated by a variety of factors, including tubing length and the type, number and configuration of the hard plastic components: ports, connectors, clamps and drip chambers.
John Richard, Vice president and general manager of DuPont’s Safety business
The solution
An employee-led “Green Team” at B. Braun is responsible for embracing, employing and elevating the company’s sustainability objectives in different functions, and packaging is no exception. B. Braun’s packaging team evaluated an array of materials to streamline the overall packaging size. In reviewing the existing package configurations, it was found that reorienting the infusion set components within the package could achieve a significantly reduced packaging footprint. However, a more compact package increased the probability of friction, tear and abrasion from the rigid components, and minimizing the coil size could lead to kinking the flexible components.
All these risks are exacerbated by shipping/distribution conditioning, so selecting the right materials for the application was key. In addition, the materials needed to be compatible and meet the manufacturing demands of the form-fill seal environment. To meet these product and manufacturing requirements, the team conducted an extensive evaluation of a variety of materials before landing on a solution that provides the right balance of strength, durability and manufacturability. For top web, uncoated Tyvek® 1059B from DuPont enabled a more compact design while ensuring no compromise in puncture and abrasion resistance, thereby preserving product functionality as well as sterile integrity.
In addition, use of uncoated Tyvek® helped deliver a less processed packaging solution, creating efficiencies in the supply chain. For the bottom web, a formable coextruded nylon film structure was selected. It too provided adequate protection to the product, and manufacturability while enabling a clean peel with uncoated Tyvek®. The processability of the material combination achieved a desired sealing window via upgrading sealing stations, controllers and upfront design provisions to include a seal skirt (unsealed area around the perimeter of the package) which helped ensure a clean peel and reduced chances of fiber tear. Finding this solution was not an easy process. It was enabled through a collaborative, iterative process of product and packaging engineering. The team’s efforts delivered a more robust package design with reconfigured component placement, a stronger bottom web, anchored in Tyvek® as the top web.
The impact
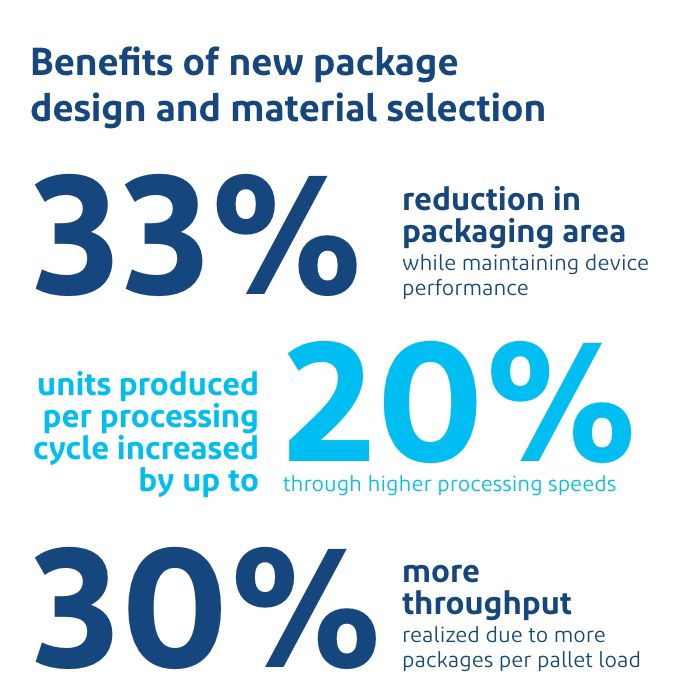
The new package design as well as materials selection resulted in significant benefits across all aspects of the packaging lifecycle. A stronger bottom web/Tyvek® top web material combination enabled up to 33% reduction in packaging area while maintaining device performance. The number of units produced per processing cycle increased by up to 20% through higher processing speeds, along with productivity savings through shorter changeover times and increased shelf space in the inventory. Further, the streamlined package design unlocked additional capacity by increasing the number of units produced, boosting ROI. Up to 30% more throughput was realized due to more packages per pallet load, while ensuring no compromise on sterile integrity.
Increased packing density resulted in lower transportation and distribution costs with success in transportation and distribution testing. The package provided best-in-class abrasion and puncture resistance, minimizing probability of fiber tear events and retaining the general look and feel of the package, which was important to users. Finally, optimization of primary packaging design, coupled with reduction in secondary packaging, resulted in reducing packaging waste, a feature that won tremendous appreciation from nurses.
“DuPont is proud of the collaboration with leading healthcare company B. Braun Medical Inc. to design a Tyvek® solution that addressed industry and consumer needs while having a positive and measurable impact on our shared sustainability goals – this is a prime example of how we strive to partner and solve for challenges in a creative and innovative way,” said John Richard, vice president and general manager of DuPont’s Safety business