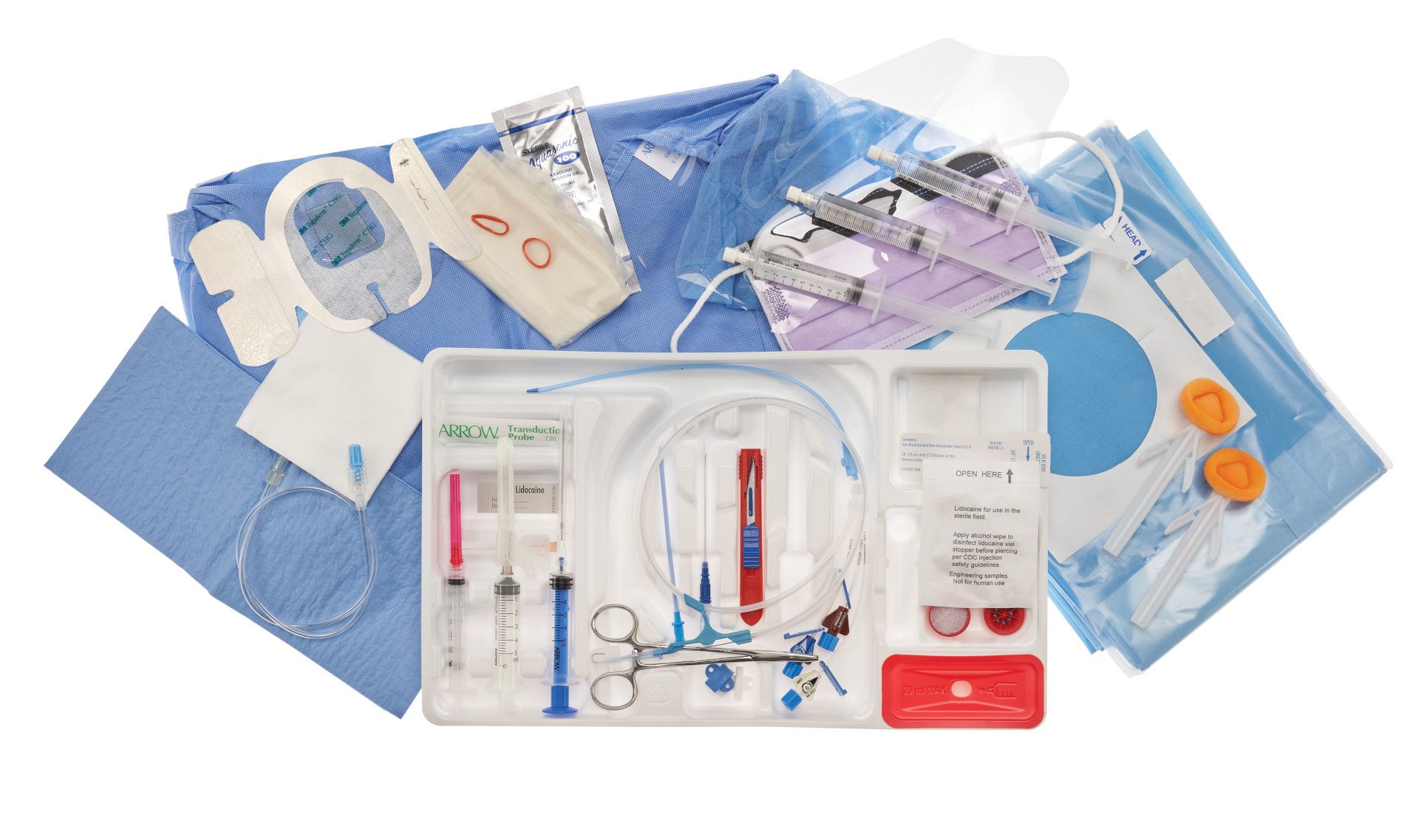
Driven to continuously improve operational excellence, The Global Packaging Group at Teleflex Incorporated (Teleflex), undertook a package redesign project to improve manufacturability and sustainability of its Central Venous Catheter (CVC) Arrow® ErgoPack® Complete System product package. The DuPont Tyvek® Healthcare Packaging team is pleased to share more about this project and highlight how the team at Teleflex made this concept a reality.
The challenge—updating a legacy package design
Like many other medical device manufacturers (MDMs) of large kit products, Teleflex relied on a preformed rigid tray and coated lid made of Tyvek® for its CVC tray products; and rightfully so, because some of the kits weigh more than 1 kg (2.5 lb). Furthermore, healthcare personnel worldwide are familiar with this packaging configuration.
Encasement within the tray greatly reduces the risk of product damage while the superior strength, porosity, and microbial barrier of Tyvek® make it the “go-to” option for medical devices. Lids made of Tyvek® and rigid tray configurations have been relied upon for years to help safely deliver products and protect patient safety.
As the product line grew, Teleflex identified packaging process improvement opportunities and lifecycle efficiencies that needed further exploring.
The packaging process of the legacy design was laborious and operator dependent. First, each kit was wrapped in a sterile wrap and placed into a tray. The filled trays were then loaded into the sealer and a printed lid made of Tyvek® was placed on the tray. Finally, the sealing operation to affix the lid to the tray created the finished sterile barrier package.
After this, secondary packaging processes ensued, as threeand five-pack cases were assembled. Corrugated dividers were placed between each tray in all configurations and 80% of the kit trays utilized foam corner protectors.
The packaging operation, specifically the tray sealing process, became a bottleneck in the manufacturing operations, prompting the team to kick off a project to modernize and develop a more sustainable, streamlined process.
Using the lifecycle of a medical package, we will discuss how the Teleflex team achieved the goal of developing a package design that improves both manufacturability and sustainability.
The solution—package design of the future

Package development
The Teleflex team looked to a form-fill-seal (FFS) packaging process to replace the current design and process; however, given the number of configurations and the overall weight and size of its kits, moving to an FFS package design presented unique challenges.
One of the most significant changes the team pursued was the transition from a rigid tray to a flexible tray, introducing not only an evolution in the package design and performance, but also the user perspective and experience.
Going from a rigid to flexible tray was quite a radical change for this application. Not only would the package design need to meet the distribution and handling requirements, but it also needed to be accepted by healthcare end users.
Selecting materials
On average, the kits measure 46 cm x 31 cm (18” x 12”) and have depths of about 6 cm, 9 cm and 10 cm (2.5”, 3.5” and 4”). From its sheer size and weight, the product is not a typical candidate for an FFS package. With tooling designed for the large footprint package, the Teleflex team sought out bottom web materials that could form the deep trays and a top web that would deliver the needed performance in this new, flexible package design.
Following extensive evaluations and trials of various materials for the bottom web, the team chose a coextruded nylon/polyethylene (PE) film structure. The selected film structure formed to the needed draw depth while maintaining a wall thickness capable of withstanding the challenges of distribution and product use.
For the top web, the Teleflex team chose to remain with a coated Tyvek® 1073B because it paired well with the thicker forming film and has superior strength and durability to address the demanding challenges of the application. The strength and durability properties of Tyvek® helped enable it to resist tearing and puncture while its porosity and outstanding microbial barrier performance make it the proven go-to material for many MDMs and the products they need to protect.

Image courtesy of Teleflex.
Manufacturing
Benefits to supply chain and procurement
Switching from preformed packaging components to sourcing roll stock simplified the procurement process and drove numerous supply chain improvements.
For example, shifting converting operations to FFS for this application helped to reduce packaging material lead times and overall costs.
In addition, shipment of roll stock materials was more efficient than shipping converted components. By moving to the roll stock format, they reduced the number of shipments needed to transport packaging materials by more than 90%—by liminating more than 1,000 pallets.
What’s more, incoming inspection and acceptance of materials into inventory was simplified and less labor intensive.
FFS process improvements
Transitioning to FFS from the manual tray sealing process resulted in a variety of improvements, efficiencies and ultimately reduced risk across the manufacturing process.
The legacy packaging operation was dependent on manual assembly and sealing processes. Inherently, these manual procedures are influenced by human operators and high levels of attention and inspections were required on the highvolume product lines to avoid sealing and quality challenges. By moving the process to the automated FFS lines, these risks were eliminated, contributing to a more resilient and efficient manufacturing operation.
With the rotary sealers, changeover between tray sizes was a time-consuming and umbersome process because sealing nests and process parameters needed to be adjusted. The FFS lines eliminated this downtime because there is no need to change sealing parameters and changing the forming mold depth on the trays takes less than a minute. Thus, the FFS lines successfully eliminated the sealing process bottleneck that Teleflex was experiencing with the legacy packaging operation.
FFS lines improved operational throughput, enabling Teleflex to produce more products and increase share in growth markets. Continued growth will be driven by expanding existing product lines and creating a new packaging platform.
The transition of both the package format and the manufacturing process helps to demonstrate the adaptability and functionality that Tyvek® brings to MDMs. In both the design and process development, Tyvek® was an integral part of this packaging solution. While other materials and processes changed, Tyvek® helps ensure that the packaging system will be robust and endure the challenges of the product lifecycle. From manufacturing to sterilization and distribution, Tyvek® packaging addresses these demands and helps protect patient safety.
Sterilization
The manufacturing and process flow of the products allowed the packaging change to be made without modifications to the sterilization process. This allowed the packaging team to narrow the project scope to focus on and change only the packaging configuration. However, it is important to note that the sterilization process could gain efficiency improvements when more products can be included in the same sterilization cycle.

Distribution
With this new FFS package design, the Teleflex team was able to achieve transportation efficiencies while also reducing the risk of damage during shipment.
Optimizing corrugated case sizes led to significant transportation efficiencies through improved pallet configurations. In fact, pallet reconfiguration enabled an average increase of 27% more products per pallet in 90% of the different kit configurations. These efficiencies eliminated the shipment of 1,860 pallet loads per year.
The robust sterile barrier package design was key to realizing these efficiencies while also reducing the risk of damage incurred during shipment. With its ability to endure the challenges and hazards of transportation, Tyvek® can help reduce the risk of product damage, thereby reducing distribution costs and improving the overall sustainability of the product.

Product use
Transitioning from a rigid tray package to the flexible package is a noticeable change when it comes to the user experience. Many healthcare professionals are accustomed to sealed rigid trays for these types of products and the packaging team recognized they were challenging this norm.
To gauge market acceptance and adoption, the team conducted market research of the usability aspects of different packaging formats used in the healthcare setting.
Initially, the team was met with some pushback and reservations on the design; however, after learning more and having hands-on experience with the flexible package, end users began to accept the design and started to recognize the additional benefits it would present in the clinical setting.
For example, the flexible package fits better in carts and trays used to store the products. Although the overall volume of space the legacy package required was driven by the size of the inflexible tray, the flexible nature of the new design enables more efficient storage. Aiding the storage improvement, Tyvek® delivers the needed product protection in clinical environments that often present unique challenges and potential risks to sterile products.
Another benefit is that the flexible nature of the overall package reduces the amount of space the empty package takes up in waste collection.

Sustainability
Primary (SBS) and secondary packaging material improvements*
The new FFS package design reduced the sterile barrier system (SBS) packaging weight by an average of 67%, leading to an avoidance of nearly 120 tonnes of plastic packaging on an annual basis.
Eliminating the foam protectors that were used in 80% of the kit trays removed an additional 10 tonnes of plastic from the packaging system. And with the foam protectors removed, the case sizes of these kits could be optimized, reducing the footprint of the case by up to 24% and yielding up to a 72% reduction in corrugated consumption by weight.
By optimizing the case sizes and eliminating the need for the corrugated dividers, nearly 400 tonnes of corrugated board were removed from the system.
The elimination and reduction of packaging materials enabled the project to net significant carbon dioxide (CO2) reductions totaling >710,000 kg (>1.5 million lb) annually.

* The material usage and improvement data are based on estimated annual volumes provided for this study. The CO2 savings have been calculated based on these estimated annual volumes and using data sources from Ecoinvent 3.8 and from Eastman data published in 2013.
Impact
Teleflex achieved its goal of improving both the manufacturability and sustainability of its Arrow® ErgoPack® Complete System product packaging by switching to an FFS packaging process. This was enabled by retaining the strength and microbial barrier performance of the package through the use of Tyvek® 1073B as top web material.
Specifically, Teleflex:
- Avoided >710,000 kg (>1.5 million lb) of CO2.
- Reduced packaging material lead times and overall costs while retaining performance.
- Simplified inspection and acceptance of materials into inventory.
- Eliminated downtime associated with the previous manufacturing process.
- Increased share in growth markets through expansion of existing product lines.
- Introduced new 11 cm and 12 cm (4.5” and 5”) FFS packages.
