DuPont™ Tyvek® Supports In-Hospital Decontamination of Used N95 Respirator Masks
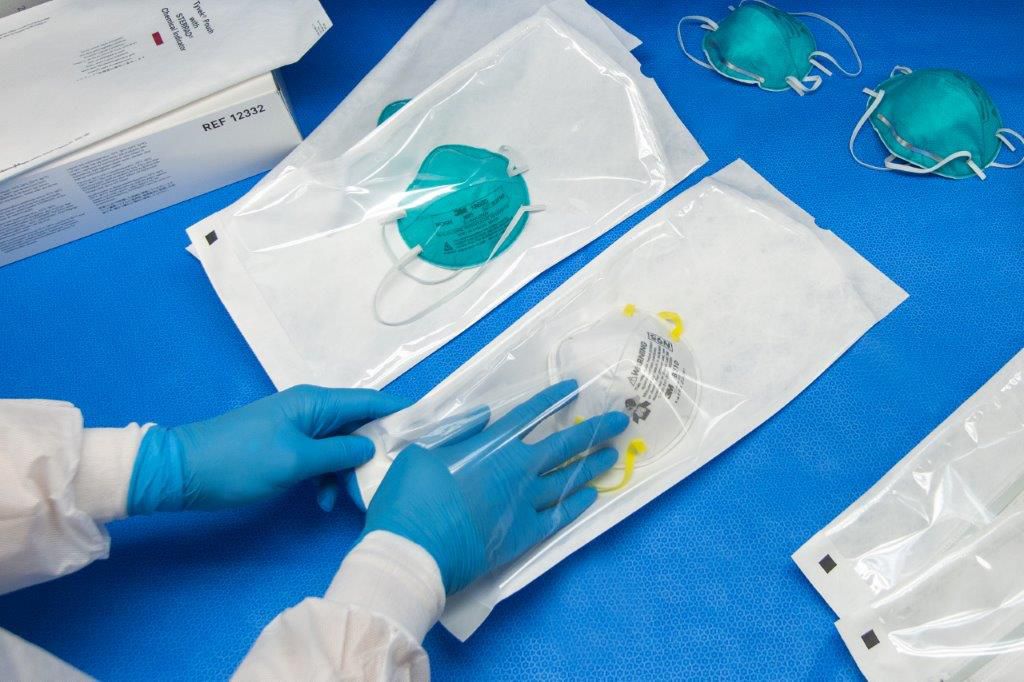
The rapid increase in COVID-19 cases around the world has created a severe shortage of critically needed personal protective equipment (PPE), especially N95 respirator masks. While these respirators were originally designed to be used only once for a single patient, these shortages have driven health care providers and frontline workers to desperate circumstances in which they are reusing or self-sanitizing respirators. News stories have even shown examples of workers hiding supplies of respirators in their lockers and using Lysol® spray in desperate measures to protect themselves.
To help resolve this critical issue, several companies have been quickly working to combine existing sterilization technologies with innovative thinking to develop new methods for effectively decontaminating used respirators after each use. To support the in-hospital decontamination process, DuPont also recognized the need to expedite supply of Tyvek® pouches for these applications. In support of these decontamination technologies, the U.S. Food and Drug Administration (FDA) issued several Emergency Use Authorizations (EUA) to companies such as Advanced Sterilization Products (ASP), STERIS, Battelle, Sterilucent and Stryker.[1]
These companies have wasted no time in acting on these emergency use authorizations. For example, Advanced Sterilization Products’ (ASP) protocol for decontaminating select N95 masks has been shown to triple the lifespan of masks by allowing a single-use mask to be decontaminated two additional times. By using ASP’s STERRAD® Sterilizers which are already in many healthcare facilities, this process offers the ability to reprocess up to 480 masks per STERRAD® Sterilizer machine daily. “We are committed to provide support to the medical community and continue to work closely with our value chain partners to implement this new protocol to help keep our health care professionals heroes safer as they remain at the front-lines in the battle against COVID-19,” said Dominic Ivankovich, President of ASP.[2]

STERIS is another company also demonstrating quick results following the FDA authorization. Walt Rosebrough, President and Chief Executive Officer of STERIS noted, “Healthcare providers are on the front lines of this pandemic and are in desperate need of personal protective equipment. We are pleased to be able to offer a partial solution for healthcare providers during this crisis and hope that this temporary authorization will provide some relief to them.” The STERIS process can decontaminate 10 masks per cycle and each mask can be processed up to 10 times.[3]
All of these decontamination methods approved by the FDA use some form of vapor phase hydrogen peroxide to decontaminate the N95 masks. Because vaporized hydrogen peroxide is not compatible with cellulosic materials like paper, DuPont™ Tyvek® pouches are recommended and already validated as the packaging method of choice for all methods except Battelle, which processes the masks without packaging.[4] Furthermore, pouches made with Tyvek® provide additional benefits in helping to prevent subsequent contamination of decontaminated masks during transportation, handling, or storage.
Innovation and agility have been the keys to this successful response, and DuPont successfully met the challenge head on. This new need for Tyvek® presented an opportunity for the DuPont™ Tyvek® Medical & Pharmaceutical Packaging team to quickly join the effort and collaborate with its authorized converters to meet the growing demand for Tyvek® pouches in this new application. As the pandemic and the resulting global response evolved, the healthcare team quickly secured Tyvek® supply and leveraged relationships with its converting partners who had the capabilities and capacity to expedite the manufacturing of these urgently needed pouches. This enabled delivery of much needed pouches into the hands of health care systems that were already set up with existing sterilization machinery to decontaminate used N95 masks.
These circumstances exemplify global collaboration and coordination across the healthcare industry to deliver critical supplies to health care providers and frontline workers battling the COVID-19 pandemic. DuPont is proud of these efforts and partnerships, and its dedicated team will continue to strive every day to deliver the urgently needed supplies of Tyvek® Medical & Pharmaceutical Packaging to the COVID-19 response.
References
This information is based on technical data DuPont believes to be reliable. It is subject to revision as additional knowledge and experience are gained. It is not intended as a substitute for any testing you may conduct to determine for yourself the suitability of our products for your particular purpose. Since conditions of use are outside the Company’s control, DuPont makes no warranties, express or implied, and assumes no liability in connection with any use of this information. DuPont™, the DuPont Oval Logo, and all trademarks and service marks denoted with TM, SM or ® are owned by affiliates of DuPont de Nemours, Inc. unless otherwise noted. © 2020 DuPont. (04/20)